Abstract
Background Acute bleeding leads to significant morbidity and mortality. Coagulopathy during acute bleeding further increases the risk of death. Recent studies have shown that the increase in activated protein C (APC) is one of the major contributors to coagulopathic conditions. Recombinant Factor VIIa (rFVIIa) had been reported to have some therapeutic effects in some clinical trials, however, its use was associated with thromboembolic events. Therefore, we sought to develop a novel FVIIa molecule (CT-001) with enhanced activity and lower thrombogenicity to address these bleeding situations. CT-001 has 4 N-glycans (T106N/N145/V253N/N322) with terminal sialic acid removed to promote active clearance via the asialoglycoprotein receptor, and P10Q/K32E substitutions introduced to its Gla-domain for enhanced phospholipid affinity and activity.
Aim We aim to characterize the pro-hemostatic activity of CT-001 in coagulopathic conditions mediated by APC.
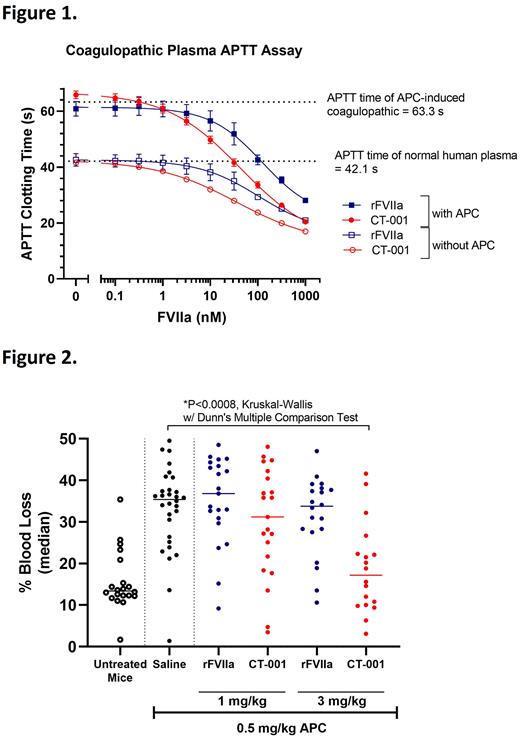
Results An APTT-APC clotting assay was used to evaluate the procoagulant activity of CT-001 and rFVIIa in plasma rendered coagulopathic by APC. 2.25 nM APC was spiked into the normal plasma to induce a coagulopathic condition and prolong APTT clotting time by 1.5x fold (from the normal of 42 s to 63 s). 31.6 nM CT-001 corrected the APTT time of coagulopathic plasma back to normal (Fig 1). In comparison, a supra-therapeutic concentration of 100 nM rFVIIa was needed. In a thrombomodulin-modified thrombin generation assay (TM-TGA), CT-001 also restored peak thrombin and endogenous thrombin potential (ETP) at a dose lower than rFVIIa. Based on these observations, we next tested the efficacy of CT-001 in mice with coagulopathic bleeding induced by APC. In an APC-mediated coagulopathic hemorrhage mouse model with tails amputated 4 cm from the tip, 3 mg/kg CT-001 reduced blood loss significantly whereas minimal reduction was observed with rFVIIa (Fig 2). The efficacy of CT-001 was further tested in a coagulopathic saphenous vein bleeding model. In this model, mice were treated with activated protein C to induce coagulopathy. The saphenous vein was injured to initiate bleeding. The injury was treated with CT-001 or rFVIIa. After the cessation of the first bleed, the bleeding time was recorded, and the clot was disrupted to initiate a new bleeding episode for a second bleeding time measurement. This process was repeated until 30 mins had elapsed after test articles were administrated. The number of clot formations and duration of bleeding episodes during the 30 min period were collected. In untreated coagulopathic mice, the median number of clot formations within a period of 30 mins was 2.5, and the median clotting time of each bleed was 681s. 3 mg/kg CT-001 provided a significantly higher pro-hemostatic effect and increased the median number of clot formations to 31, while the median clotting time of each bleed was reduced to 25 s. In comparison, 3 mg/kg rFVIIa treated mice had only 6 clots formations and the median clotting time of each bleed was 89s.
Conclusions Collectively, these data show that CT-001 has the potential of providing enhanced pro-hemostatic activity to counteract APC pathway-mediated coagulopathic conditions and reduce coagulopathic blood loss in acute bleeds.
Disclosures
Sim:BMS: Other: Spouse's employment; Coagulant Therapeutics: Current Employment, Current equity holder in private company. Mallari:Coagulant Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Bauzon:Gladiator Biosciences: Consultancy, Current equity holder in private company; Catalent Biologics: Current Employment; Coagulant Therapeutics: Consultancy, Current equity holder in private company, Patents & Royalties: Patent. Hermiston:Jazz Pharmaceuticals: Consultancy; Coagulant Therapeutics: Current Employment, Current equity holder in private company, Patents & Royalties: Patent; Gilead: Consultancy; Kalivir Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Gladiator Biosciences: Current Employment, Current equity holder in private company, Patents & Royalties: Patents; SoBi: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.